A Trial of Three Rounds of Mass Drug Administration with Azithromycin for Yaws
John LN, Beiras CG, Houinei W, Medappa M, Sabok M, Kolmau R, Jonathan E, Maika E, Wangi JK, Pospíšilová P, Šmajs D, Ouchi D, Galván-Femenía I, Beale MA, Giacani L, Clotet B, Mooring EQ, Marks M, Vall-Mayans M, Mitjà O. Trial of Three Rounds of Mass Azithromycin Administration for Yaws Eradication. New England Journal of Medicine. 2022 Jan 6;386(1):47-56. PMID: 34986286.
Abstrak
Latar Belakang: Treponema pallidum subspesies pertenue menyebabkan penyakit yaws atau frambusia. Strategi dilakukan untuk pengendalian yang lebih baik dan diharapkan dapat mengeliminasi frambusia jika diperlukan.
Metode: Peneliti melakukan uji klinis acak klaster yang open-label di daerah endemik frambusia wilayah Papua Nugini. Tiga puluh delapan area diacak untuk menerima salah satu dari obat massal (Mass Drug Administration/MDA) yang diikuti oleh dua putaran targeted treatment kasus aktif (kelompok kontrol) atau tiga putaran MDA (kelompok eksperimen) dengan interval 6 bulan. Perbedaan prevalensi frambusia aktif dan laten dinilai pada survei 18 bulan.
Hasil: Sembilan belas area (30.438 orang) diacak ke dalam kelompok kontrol dan 19 (26.238 individu) ke dalam kelompok eksperimen. Sebanyak 24.848 dosis azithromycin diberikan pada kelompok kontrol (22.033 pada awal penelitian, 207 peserta dengan lesi seperti frambusia dan 2.608 kontak pada 6 bulan dan 12 bulan), dibandingkan dengan 59.852 dosis pada kelompok eksperimen.
Setelah 18 bulan, prevalensi frambusia aktif turun dari 0,46% (102/22.033) menjadi 0,16% (47/29.954) pada kelompok kontrol dan dari 0,43% (87/20.331) menjadi 0,04% (10/25.987) pada kelompok eksperimen (RR 3,54; 95% CI 1,72–7,27).
Prevalensi dari ulkus infeksi menular lainnya menurun pada tingkat yang sama pada kedua kelompok. Prevalensi frambusia laten pada 18 bulan follow-up yang dinilai pada 994 anak di kelompok kontrol dan 945 anak di kelompok eksperimen adalah 6,54% (5,00–8,08) dan 3,28% (2,14–4,42) (RR 2.03; 1.12–3.7). Tiga kasus dengan resistansi terhadap makrolida ditemukan dalam penelitian.
Kesimpulan: Data penelitian ini menunjukkan bahwa MDA azithromycin tiga putaran dengan selang waktu 6 bulan lebih baik daripada MDA azithromycin satu putaran yang diikuti dengan dua putaran targeted treatment untuk mengurangi prevalensi frambusia pada komunitas. Pemantauan kemunculan dan penyebaran resistansi antimikroba perlu dilakukan.[1]
Ulasan Alomedika
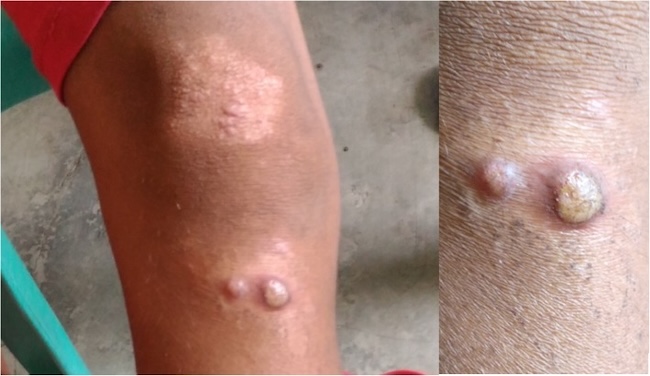
Frambusia adalah infeksi kulit dan tulang yang disebabkan oleh Treponema pallidum subspesies pertenue. Penyakit ini banyak ditemukan pada lingkungan dengan ekonomi rendah di kawasan lembab dan tropis di Afrika, Asia, Amerika Latin dan Pasifik, dengan usia terdampak 75–80% adalah anak-anak di bawah 15 tahun.[2]
Pada tahun 2021, sebanyak 123.866 kasus dilaporkan dari 13 negara dan 1.102 kasus terkonfirmasi dari 9 negara. Lebih dari 80% kasus dilaporkan di wilayah Pasifik Barat, khususnya Papua Nugini, meskipun mayoritas kasus di wilayah ini belum dikonfirmasi secara laboratorium. Tanpa adanya eradikasi, frambusia diperkirakan menyebabkan 1,6 juta kasus disabilitas antara tahun 2015–2050.[3,4]
Beberapa penelitian telah membuktikan bahwa pemberian azithromycin dosis tunggal efektif untuk tata laksana frambusia. Namun, terdapat juga penelitian yang melaporkan adanya relaps. Penelitian ini dilakukan untuk mengevaluasi apakah MDA azithromycin tiga putaran lebih efektif daripada terapi standar dengan MDA satu putaran saja.
Ulasan Metode Penelitian
Penelitian ini dilakukan mulai dari April 2018 hingga Oktober 2019 pada area endemis frambusia di Papua Nugini. Randomisasi dilakukan pada 38 area dari 3 bagian distrik. Sebanyak 19 area (30.438 orang) masuk ke kelompok kontrol yang menerima strategi Morges, yaitu MDA tunggal dengan azithromycin pada awal penelitian, diikuti dengan dua siklus targeted treatment pada bulan ke-6 dan ke-12. Sementara itu, 19 area lainnya (26.238 orang) masuk ke kelompok eksperimen dan menerima tiga siklus MDA azithromycin pada awal penelitian, bulan ke-6, dan ke-12.
MDA terdiri dari pengobatan menyeluruh tanpa memandang status infeksi. Sedangkan targeted treatment terdiri dari skrining proaktif dari semua partisipan dengan adanya bukti frambusia, dan adanya kontak dengan keluarga atau teman sekolah.
Azithromycin diberikan sebagai dosis oral tunggal dengan observasi secara langsung. Dosis disesuaikan dengan berat badan terhadap usia yang direkomendasikan oleh pedoman WHO, yaitu: usia ≥15 tahun (4 tablet 500 mg), usia 10–14 tahun (3 tablet), usia 5–9 tahun (2 tablet), usia 1–4 tahun (1 tablet atau 7,5 mL sirup azithromycin). Sirup azithromycin (5 mL) diberikan untuk anak usia 1–12 bulan.
Luaran primer penelitian ini adalah prevalensi frambusia aktif yang terkonfirmasi dengan PCR pada seluruh populasi penelitian, dan prevalensi serologis frambusia laten yang dinilai dari anak-anak asimtomatik usia 1–15 tahun dalam masa survey 18 bulan. Peneliti juga menilai prevalensi frambusia aktif pada awal penelitian, 6 bulan, dan 12 bulan.
Ulasan Hasil Penelitian
Populasi penelitian terdiri dari 56.676 orang yang tinggal di 38 area yang secara acak dibagi menjadi kelompok kontrol (19 area, 30.438 individu) atau kelompok eksperimen (19 area, 26.238 individu). Sebanyak 42.362 (74,7%), 36.810 (64,9%), 48.488 (85,6%) individu menerima intervensi (MDA atau targeted treatment) pada awal penelitian, 6 bulan, dan 12 bulan.
Jumlah dosis azithromycin yang diberikan secara keseluruhan adalah 24.848 pada kelompok kontrol (22.033 di awal penelitian, 207 peserta dengan lesi seperti frambusia, dan 2.608 kontak pada siklus targeted treatment 6 bulan dan 12 bulan), dibandingkan dengan 59,852 di kelompok eksperimen. Total individu yang dapat dinilai setelah 18 bulan adalah sebanyak 55.941 (98,7%).
Selama penelitian, peneliti mengidentifikasi 1.026 ulkus, di mana 297 di antaranya dikonfirmasi PCR sebagai frambusia. Pada awal penelitian, prevalensi frambusia aktif adalah 0,46% (102/22,033) di kelompok kontrol dan 0,43% (87/20,331) di kelompok eksperimen. Pada survei 18 bulan, prevalensi frambusia aktif turun menjadi 0,16% (47/29,954) dan 0,04% (10/25,987) pada kelompok kontrol dan eksperimen (RR 3,54; 95%CI 1,72–7,27).
Data penelitian tersebut menunjukkan bahwa strategi pemberantasan frambusia harus mempertimbangkan siklus MDA tambahan. Pemberian azithromycin 3 putaran dengan selang waktu 6 bulan lebih efektif menurunkan angka frambusia dibandingkan dengan pemberian MDA azithromycin satu siklus saja dilanjutkan dengan targeted therapy.
Kelebihan Penelitian
Penelitian ini memiliki beberapa keunggulan. Pertama-tama, desain cluster-randomized memberikan bukti bahwa penurunan prevalensi frambusia aktif dan laten disebabkan oleh intervensi eksperimental.
Kedua, generalisasi penemuan dari subdistrik yang terletak di wilayah yang luas dan berdekatan dengan wilayah subdistrik yang tidak mendapat terapi lebih tinggi daripada penelitian sebelumnya di kepulauan kecil. Selain itu, intervensi dilakukan dalam struktur sistem kesehatan rutin dengan sumber daya lokal di tingkat masyarakat dan dipimpin oleh Departemen Kesehatan Nasional.
Keterbatasan Penelitian
Terdapat beberapa keterbatasan dalam penelitian ini termasuk tidak adanya sensus penduduk yang diperbarui. Selain itu, peneliti tidak dapat mengetahui apakah jumlah putaran MDA yang berbeda akan lebih efektif. Hal ini akan terjawab jika jumlah kasus pada kelompok eksperimen lebih rendah 6 bulan setelah MDA terakhir, tetapi hal ini tidak dapat dilakukan karena adanya pembatasan perjalanan akibat COVID-19 saat itu. Selain itu, penelitian ini tidak menilai resistansi makrolida pada organisme lain.
Aplikasi Hasil Penelitian di Indonesia
Penyakit frambusia masih menjadi masalah kesehatan di Indonesia. Sampai tahun 2022, sebanyak 74 kabupaten masih tergolong endemis frambusia. Upaya eradikasi telah dilakukan sesuai Permenkes Nomor 8 Tahun 2017 tentang Eradikasi Frambusia, termasuk dengan pemberian obat pencegahan massal berupa azithromycin dengan dosis 30 mg/kgBB (maksimal 2 gram) dan survei serologi.[5,6]
Hasil penelitian ini memberikan bukti tambahan bahwa untuk mengurangi prevalensi frambusia, pemberian obat pencegahan massal (MDA) berupa azithromycin tiga putaran dengan selang waktu 6 bulan lebih baik daripada MDA azithromycin satu putaran yang diikuti dua putaran targeted treatment. Temuan ini dapat diaplikasikan di Indonesia sebagai tambahan terhadap program yang sudah ada.